Consider a molecule in which groups A and B are joined by two CH2 (methylene) groups. If A and B are pulled as far apart as possible, the molecule is in its fully extended anti or staggered conformation:

Groups A and B are said to be antiperiplanar (ap) in this conformation. Not only are A and B as far apart as possible but also all of the hydrogen atoms are at their maximum distances one from the other. This can be seen by viewing the molecule down the axis joining the carbon atoms (Newman projection). Rotation of the second carbon atom 180° around the single bond yields the eclipsed conformation in which groups A and B are synperiplanar.
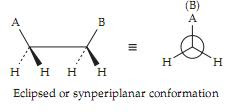
If A and B are large bulky groups they will bump together, attainment of the eclipsed conformation will be almost impossible, and rotation will be severely restricted. Even if A and B are hydrogen atoms (ethane), there will be a rotational barrier in the eclipsed conformation which amounts to ∼12 kJ (3 kcal) per mole because of the crowding of the hydrogen atoms as they pass each other. This can be appreciated readily by examination of space-filling molecular models.
If groups A and B are methyl groups (butane), the steric hindrance between A and B leads to a rotational barrier of ∼25 kJ ( 6 kcal) per mole. The consequence of this simple fact is that in fatty acids and related substances and in polyethylene the chains of CH2 groups tend to assume fully extended zigzag conformations.
In addition to this extended conformation there are two gauche (skewed or synclinal) conformations which are only slightly less stable than the staggered conformation and in which A and B interfere only if they are very bulky. In one of the twogauche conformations B lies to the right of A and in the other to the left of A when viewed down the axis.

These two conformations are related to right-handed and left-handed screws, respectively. The threads on an ordinary right-handed household screw, when viewed down the axis from either end, move backward from left to right in the same fashion as do the groups A and B in the illustration. The angle φ is the torsion angle and is positive for right-handed conformations. Gauche conformations are important in many biological molecules; for example, the sugar alcohol ribitol stacks in crystals in a “sickle” conformation, in which the chain starts out (at the left) in the zigzag arrangement but shifts to a gauche conformation around the fourth carbon atom, thereby minimizing steric interference between the OH groups on the second and fourth carbons.

The complete series of possible conformations is shown
in Fig 1.

Figure 1. Description of conformations about a single bond in the terminology of Klyne and Prelog10,11 using the Newman projection. Group A is on the front atom at the top: the conformation is given for each possible position of group B on the other atom.
In the chain of methylene units, the hydrogen atoms on alternate carbon atoms of the fully extended chain barely touch but larger atoms cannot be accommodated. Thus, when fluorine atoms of van der Waals radius 0.135 nm replace the hydrogen atoms of radius 0.12 nm, a fully extended chain is no longer possible. For this reason the torsion angle in polyfluoroethylene is changed from the 180° of polyethylene to 166°, enough to relieve the congestion but not enough to cause severe eclipsing of the fluorines on djacent carbons. The resulting helical structure is reminiscent of those occurring in proteins and other biopolymers. We see that helix formation can be a natural result of steric hindrance between groups of atoms.

Комментариев нет:
Отправить комментарий